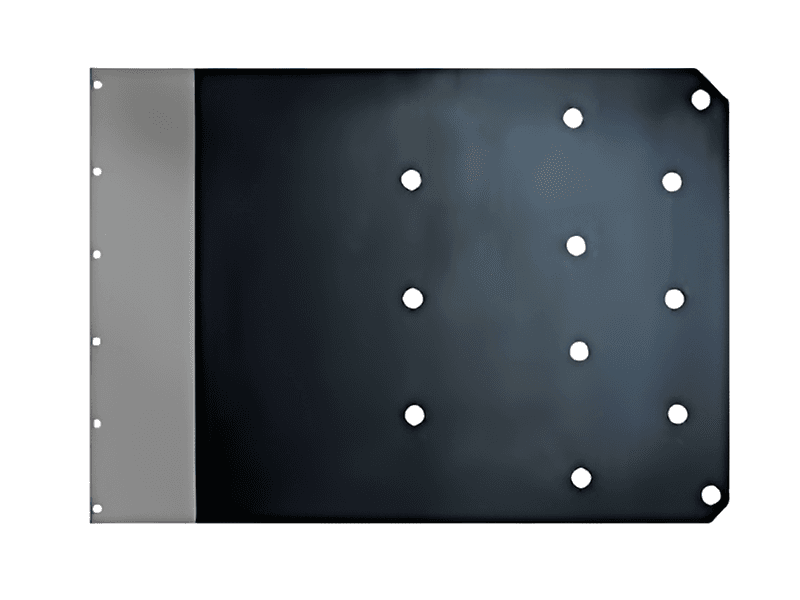
Titananode zur Kupferrückgewinnung aus Ätzlösung
Die Titananode zur Kupferextraktion aus Ätzlösungen ist ein effizientes und langlebiges elektrochemisches Material, das hauptsächlich zur elektrolytischen Rückgewinnung von Kupfer aus Ätzabfällen in der Leiterplatten- und Elektronikindustrie eingesetzt wird. Ihr Kern besteht aus einem Titanmetallsubstrat und einer oberflächenaktiven Beschichtung, die folgende Vorteile bietet:
1. Starke Korrosionsbeständigkeit: Das Titansubstrat weist eine ausgezeichnete Stabilität in sauren Ätzlösungen (wie Kupfersulfat- und Kupferchloridsystemen) auf, wodurch das Problem der Lösungsverschmutzung herkömmlicher Blei-/Graphitanoden vermieden wird.
2. Effiziente katalytische Beschichtung: Die Oberfläche ist mit einer Mischschicht aus Edelmetalloxiden wie Iridium und Ruthenium beschichtet, wodurch das Überpotential der Sauerstoffentwicklung deutlich reduziert, die Stromeffizienz verbessert (bis zu 95% oder mehr) und der Energieverbrauch gesenkt wird.
3. Langlebiges Design: Durch das spezielle Beschichtungsverfahren wird eine Lebensdauer der Anode von über 2 Jahren erreicht, wodurch die Austauschhäufigkeit reduziert wird.
4. Ökologische und wirtschaftliche Vorteile: Der Elektrolyseprozess erfordert keine Zugabe von Reduktionsmitteln und die Reinheit der Kupfergewinnung liegt über 99,9%. Gleichzeitig werden die Kosten für die Behandlung gefährlicher Abfälle gesenkt und das Ressourcenrecycling ermöglicht.
Es ist für kontinuierliche Elektrolysegeräte geeignet und wird häufig in Bereichen wie der Regeneration von Mikroätzlösungen und dem Recycling von Kupferfolien eingesetzt, mit dem doppelten Wert eines umweltfreundlichen Verfahrens und wirtschaftlichen Vorteilen.